Innovation corner

Innovation corner by Mauricio Baptista
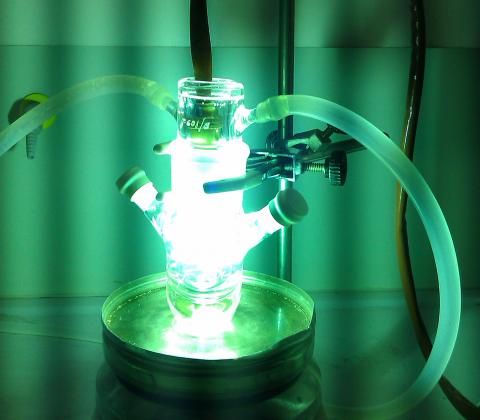
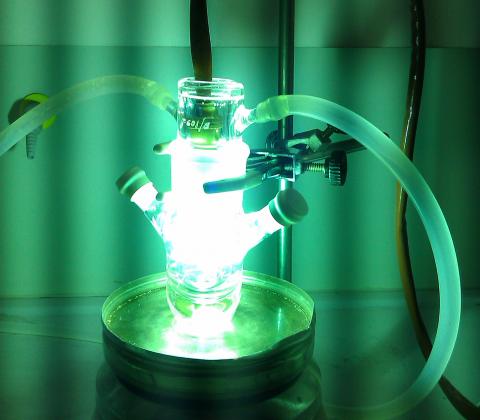
It is never too late to remind that the I in CEPID (or RIDC, in English) stands for Innovation. And the innovation has been on the rise in our Center. In fact, redox biochemistry and biology is involved in many signaling networks affecting, among other processes, homeostasis, proliferation, survival and death of cells. This brings an array of relevant basic science questions, which are closely tied to a myriad of possibilities to develop new products and new applications for chemical, cosmetic, medical and pharmaceutical industries. Indeed, CEPID team is developing several technological projects with public and private industries. Here we mention a few. In terms of the medical and pharmaceutical sectors, it is worth mentioning the development of novel drugs, including antimicrobial and anti-cancer compounds based in inorganic complexes. The use of light to treat the diabetic foot by photodynamic therapy (PDT) allowed the development of a platform of services, avoiding amputations of hundreds of patients (free-of-charge) and attracting companies working in the photonics field, including Ethik Technology and BioLambad, which are implementing their innovation strategy in partnership with RIDC Redoxoma. A larger clinical study is being designed in order to allow upscaling of possible applications and target patients. Another important health-related knowledge revealed by the Redoxoma team is the concept that visible light can damage skin and hair. As a consequence, we are developing the concept of natural pigments in sun-protection, which can decrease visible-light penetration in the skin without causing a perceived change in skin color. This line of investigation has allowed the development of joint innovation projects with several companies, including FarmaService Bioextract, Chemyunion, Johnson&Johnson and BASF. Green-chemistry catalyst is another area that has allowed transfer of technology to foster industry innovation. CEPID team developed a redox catalyst capable of performing purification of industrial wastes by Advanced Oxidation Processes. This process and the constructed reactor are being used to purify and recycle contaminated water from a petrochemical industry. Details of these results and efforts are described at our Redoxoma web page.
CEPID Redoxoma - Intellectual Property Rights
CEPID Redoxoma - Technology Transfer Developments
Mauricio da S. Baptista (baptista@iq.usp.br),
Ph.D. Professor at Department of Biochemistry,
Institute of Chemistry, University of São Paulo, Brazil
 Post image credits
Post image credits
Add new comment