Revealing the cross-talk between nitric oxide metabolites
Redoxoma Highlights, by Daniela Truzzi
Corresponding author e-mail: dtruzzi@iq.usp.br
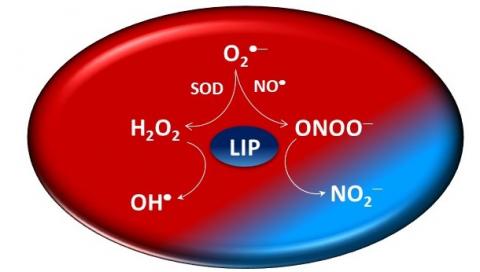
Nitric oxide (NO•) is an endogenously produced diatomic radical that regulates fundamental biological functions. Although NO• is a free radical, its reactivity in biological media is selective toward other radicals and transition metal centers. NO• metabolites include S-nitroso thiols (RSNOs), nitrite, peroxynitrite, nitrosylated heme proteins, and dinitrosyl-iron complexes (DNICs). Among these metabolites, RSNOs have gained considerable attention due to their possible involvement in NO• signaling. Biological formation of RSNO can occur by reaction of thiols with N2O3, peroxynitrite, other S-nitroso thiols (transnitrosation reactions), nitrosylated heme proteins and by a direct reaction between thiyl radicals (RS•) and NO•. Since most of these reactions are either slow or have low specificity for a signaling process, it has been proposed that S-nitrosation involves transfer of NO from DNICs to biothiols. DNICs are important NO•-metabolites, which are able to trigger vasodilation, to inhibit platelet aggregation and skin wound healing. Nevertheless, little is known about the dynamics of DNICs generation under physiological conditions. By analyzing DNIC assemble from the reaction between NO•, Fe(II) and low molecular weight biothiols (cysteine and glutathione) in aqueous media, pH 7.4, we detected mono-nitrosyl iron complex intermediate(s) and thiyl radicals (RS•) as co-products. By demonstrating that formation of DNICs yields RS• in a NO• rich environment, these results provide a novel route for S-nitroso thiol formation in biological media. Additionally, this study explains previous reports showing that DNICs and RSNOs are simultaneously formed in macrophages exposed to NO•. If such mechanism favors certain biothiols in forming RS• and thus provides specificity to RSNO formation, remains an open question. Further studies of DNICs assembly with different biothiols may contribute to answer it.
Importantly, different biothiols may react through distinct mechanism with DNICs, opening the question if all biothiols would similarly have RS• as an intermediate. This is of high relevance when considering signaling pathways and specificity.
This study involved collaboration between researchers from CEPID Redoxoma and from University of California, Santa Barbara.
Related article:
- D. R. Truzzi, O. Augusto, P. C. Ford. Thiyl radicals are co-products of dinitrosyl iron complex (DNIC) formation Chemical Communications, 55(62): 9156–9, 2019. | doi: 10.1039/c9cc04454j
Daniela Truzzi, from Department of Biochemistry,
Institute of Chemistry, University of São Paulo, Brazil

 Post image credits
Post image credits
 Post image credits
Post image credits
Add new comment