Mitochondria can dictate your fate, especially if you’re a stem cell
Redoxoma Highlights by Maria F. Forni*
Known for over a century, mitochondria have become, during the last four decades, an important subject of research within several disciplines. This is mostly due to the fact that this organelle comprises the site of oxidative phosphorylation, the citric acid cycle, fatty acid oxidation, the urea cycle and the biosynthesis of iron-sulphur centres and haem. Moreover, mitochondria are an important redox-signaling node. Indeed, the bioenergetic status of a cell is dependent on the overall quality and relative abundance of the mitochondrial population it harbors. Recent evidence suggests that the control of mitochondrial mass and morphology occurs through the processes of fusion, fission, mitophagy and biogenesis, in a tightly regulated manner known as the “mitochondrial life cycle”. It is believed that these events also play an important role in the quality-control of this organelle, and that the failure to do so could lead to several pathologies and ageing. Nonetheless, very little is known about the impact of mitochondrial dynamics in the process of stem cell differentiation.
Adult stem cells are rare, ranging in proportion from 0.1 to 5% of the total population in many mammalian tissues. They are considered the gatekeepers of tissue homeostasis since they can proliferate in order to maintain their numbers in the resident tissue but, importantly, they can differentiate and originate specialized cells, maintaining organ integrity in face of cell death imposed by ageing or injury.
Using a murine model of stromal mesenchymal stem cells derived from the skin (specifically from the dermis) we have been trying to unveil some of the alterations associated with mitochondrial homeostasis during differentiation. These cells, when properly stimulated, can generate osteoblasts/osteocytes, adipocytes and chondrocytes. It seems predictable that during differentiation an extensive metabolic reconfiguration would occur. Strikingly, we found that very early on, during the commitment period, when these cells are irreversibly specified towards a specific cell fate, the mitochondrial network is actively being remodeled. Moreover, if the processes of fusion and fusion are intentionally manipulated, the differentiation outcome can be altered. These findings demonstrate that mitochondrial morphology and its regulating processes are modulated early on during commitment, leading to alterations in the bioenergetic profile during differentiation. Our results thus suggest that mitochondrial dynamics may play a central role in the maintenance/commitment of mesenchymal stem cells.
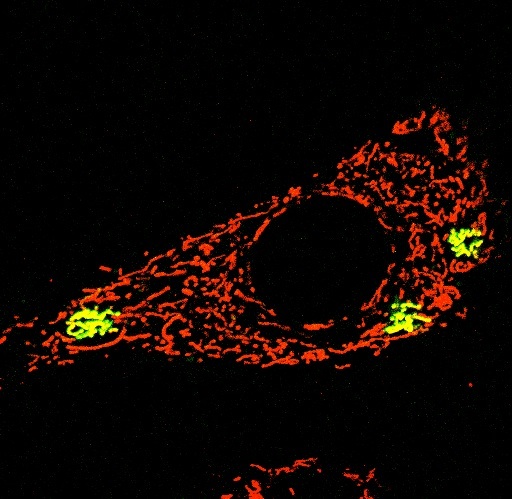
Photomicrography of a dermal mesenchymal stem cell depicting the mitochondrial network (in red, stained by TMRE) and a few individually photoactivated mitochondria (in green, stained by mito-PAGFP). Magnification 63x.[/caption]
Contributed by: Maria F. Forni, PhD.
*MF Forni is a post-doctoral fellow at IQUSP (Alicia Kowaltowski’s lab)
Department of Biochemistry, Institute of Chemistry, University of São Paulo, Brazil
Contributed by: Maria Fernanda Forni, Ph.D. *MF Forni is a post-doctoral fellow at IQUSP (Alicia Kowaltowski’s lab) Department of Biochemistry, Institute of Chemistry, University of São Paulo, Brazil
Add new comment