Redoxoma Highlights by Verônica Paviani
Oxidative modifications of proteins are extensively investigated because proteins are major targets of radicals and oxidants under physiological conditions [1]. The amino acid residues most susceptible to oxidation are the sulfur-containing residues cysteine and methionine and the aromatic residues histidine, phenylalanine, tyrosine and tryptophan. The oxidation of cysteine and methionine residues is reversible and protein-cysteine oxidation is emerging as a fundamental cell regulatory mechanism. In contrast, the oxidation of all other protein residues is irreversible, and may result in loss of protein function, protein aggregation and altered protein turnover, leading to cell and tissue dysfunction.
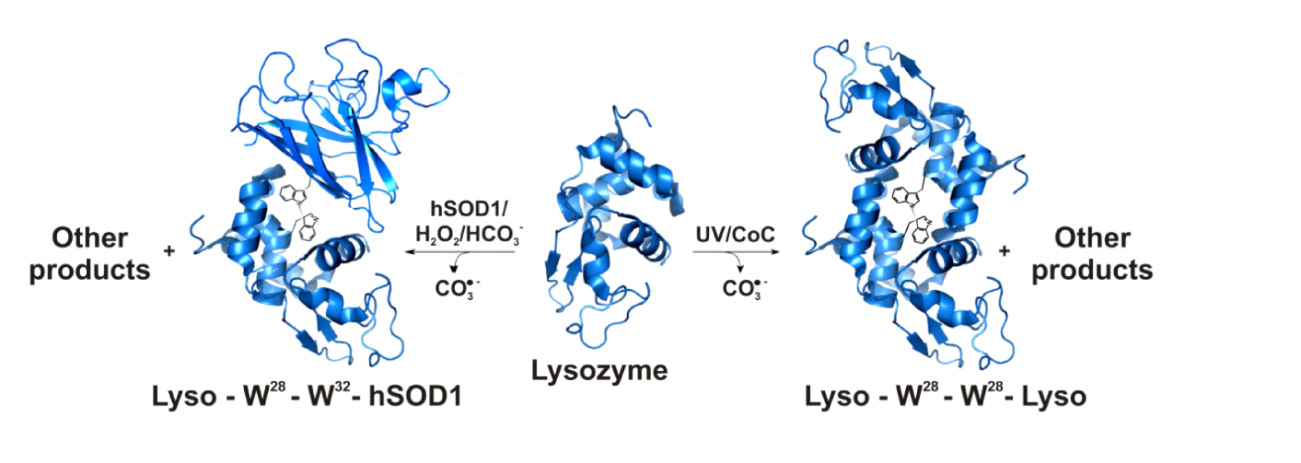
Despite the extensive investigation of the irreversible oxidations undergone by proteins in vitro and in vivo, the products formed from the oxidation of tryptophan residues remain incompletely characterized. However, protein-tryptophan residues have a unique potential to interact with other proteins and cellular structures, and their oxidation may have profound physiological consequences. For instance, our group recently characterized a novel ditryptophan cross-link caused by the recombination of human superoxide dismutase1 (SOD1)-tryptophanyl radicals, which are produced by attack of the carbonate radical (CO3•-) generated during the enzyme´s bicarbonate-dependent peroxidase activity [2]. In addition, it was demonstrated that this cross-link contributes to triggering the non-amyloid aggregation of hSOD1, a process that may be involved in the pathogenic mechanism of the nuerodegenerative disease amyotrophic lateral sclerosis [3]. Now, we show that attack of the CO3•- on lysozyme produces lysozyme and lysozyme-hSOD dimers cross-linked by a ditryptophan bond. We also show that the same lysozyme dimer is produced by UV irradiation of the enzyme [4]. In addition to confirming that the CO3•- tends to promote protein cross-links and aggregation, the results show that UV light can act similarly. Since the produced protein lesions are resistant to cellular repair, they are likely to play a role in the pathogenic mechanism of protein aggregation diseases.
- M. J. Davies. The oxidative environment and protein damage Biochimica et Biophysica Acta, 170: 93-109, 2005 | doi: 10.1016/j.bbapap.2004.08.007
- D. B. Medinas, F. C. Gozzo, L. F. A. Santos, A. H. Iglesias, O. Augusto. A ditryptophan cross-link is responsible for the covalent dimerization of human superoxide dismutase 1 during its bicarbonate-dependent peroxidase activity Free Radical Biology & Medicine, 49: 1046-53, 2010 | doi: 10.1016/j.freeradbiomed.2010.06.018
- F. R. Coelho, A. Iqbal, E. Linares, D. F. Silva, F. S. Lima, I. M. Cuccovia, O. Augusto. Oxidation of the tryptophan 32 residue of human superoxide dismutase1 caused by Its bicarbonate-dependent peroxidase activity triggers the non-amyloid aggregation of the enzyme The Journal of Biological Chemistry, 289: 30690-701, 2014 | doi: 10.1016/j.bbapap.2004.08.007
- V. Paviani, R. F. Queiroz, E. F. Marques, P. Di Mascio, O. Augusto. Production of lysozyme and lysozyme-superoxide dismutase dimers bound by a ditryptophan cross-link in carbonate radical-treated lysozyme Free Radical Biology & Medicine, in press. | doi: 10.1016/j.freeradbiomed.2015.07.015
Verônica Paviani, MS Departament of Biochemistry, Institute of Chemistry, University of São Paulo, São Paulo, Brazil.
Add new comment