Inflammatory oxidative burst: a new actor in this scenario
Redoxoma Highlights by Flavia Carla Meotti
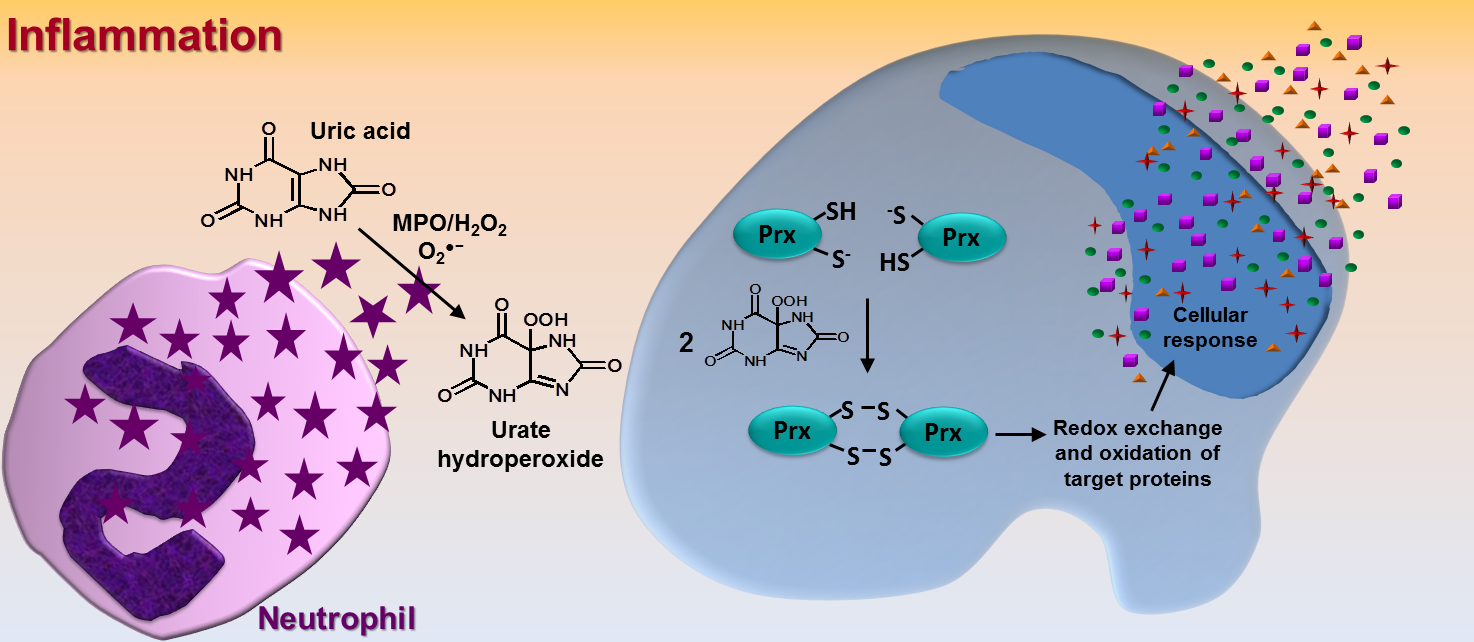
Activation of neutrophils either by invader microorganisms or by endogenous stimuli (sterile inflammation) triggers the assembling of NADPH oxidase at the membrane and the sequential production of free radicals and oxidants. This so-called inflammatory oxidative burst is initiated by the reduction of oxygen to superoxide, followed by the dismutation of superoxide to hydrogen peroxide. Hydrogen peroxide is a substrate to the heme-peroxidase, myeloperoxidase, oxidizing halides (mainly chloride) to hypohalous (hypochlorous) acid. Reaction between hydrogen peroxide and hypochlorous acid generates singlet oxygen. In parallel, the up-regulation of inducible nitric oxide synthase produces nitric oxide, which rapidly reacts with superoxide to form peroxynitrite. These are the main known players in the inflammatory oxidative burst. However, myeloperoxidase is a versatile enzyme and can effortlessly use hydrogen peroxide to oxidize other substrates in addition to halides. For instance, myeloperoxidase efficiently oxidizes uric acid to produce urate free radical, a short living reactive species that can rapidly combine with superoxide to generate an organic peroxide, urate hydroperoxide [1]. Although the formation of urate hydroperoxide was chemically feasible, the question was whether it would be biologically relevant since its precursor, urate free radical, could react with an unacountable number of biomolecules besides superoxide. This was one of the subjects addressed by our group at the Laboratory of Redox Process in Inflammation, CEPID-Redoxoma. By using mass spectrometry, we unambiguosly demonstrated the presence of urate hydroperoxide in stimulated peripheral blood neutrophils. Quantification of the amount of urate hydroperoxide that is formed by these inflammatory cells revealed that it is comparable to the amount of HOCl [2].
By analyzing the reactivity of urate hydroperoxide, we detected that it reacts preferentially with thiol groups in proteins and can rapidly oxidize the thiol peroxidases peroxiredoxin 1 (Prx1) and peroxiredoxin 2 (Prx2) [3]. These ubiquitous proteins are the fastest to react with hydrogen peroxide (~1 ⋅ 108 M-1s-1) [4] and have been proposed as hydrogen peroxide sensors, able to perform a signaling redox relay mechanism [5]. Urate hydroperoxide oxidizes purified Prx1 and Prx2 at the rate constants of 4.5 ⋅ 105 M-1s-1 and 2.3 ⋅ 106 M-1s-1, respectively, and oxidized Prx2 from intact erythrocytes at the same extent as hydrogen peroxide [3]. Together, these data suggest urate hydroperoxide as a new reported protagonist in the inflammatory oxidative burst. The oxidation of cytosolic Prx1 and Prx2 by urate hydroperoxide might affect cell function and be partially responsible for the harmfull effects attibuted to uric acid.
References
- F. C. Meotti, G. N. L. Jameson, R. Turner, D. T. Harwood, S. Stockwell, M. D. Rees, S. R. Thomas, A. J. Kettle. Urate as a Physiological Substrate for Myeloperoxidase Journal of Biological Chemistry, 286(15): 12901–11, 2011 | doi: 10.1074/jbc.m110.172460
- R. P. Silva, L. A. Carvalho, E. S. Patricio, J. P. Bonifacio, A. B. Chaves-Filho, S. Miyamoto, F. C. Meotti. Identification of urate hydroperoxide in neutrophils: A novel pro-oxidant generated in inflammatory conditions Free Radical Biology and Medicine, 126: 177–86, 2018 | doi: 10.1016/j.freeradbiomed.2018.08.011
- L. A. C. Carvalho, D. R. Truzzi, T. S. Fallani, S. V. Alves, J. C. Toledo, O. Augusto, L. E. S. Netto, F. C. Meotti. Urate hydroperoxide oxidizes human peroxiredoxin 1 and peroxiredoxin 2 Journal of Biological Chemistry, 292(21): 8705–15, 2017 | doi: 10.1074/jbc.m116.767657
- S. Portillo-Ledesma, L. M. Randall, D. Parsonage, J. Dalla Rizza, P. A. Karplus, L. B. Poole, A. Denicola, G. Ferrer-Sueta. Differential Kinetics of Two-Cysteine Peroxiredoxin Disulfide Formation Reveal a Novel Model for Peroxide Sensing Biochemistry, 57(24): 3416–24, 2018 | doi: 10.1021/acs.biochem.8b00188
- C. C. Winterbourn, M. B. Hampton. Signaling via a peroxiredoxin sensor Nature Chemical Biology, 11(1): 5–6, 2014 | doi: 10.1038/nchembio.1722
Flavia C. Meotti, Ph.D. Professor at Department of Biochemistry,
Institute of Chemistry, University of São Paulo, Brazil
Add new comment