A radical connection: Peroxynitrite and carbon dioxide

Redoxoma Highlights by by Ohara Augusto
Corresponding author e-mail: oagusto@iq.usp.br
In 2002, my laboratory published a review with the title “Nitrogen dioxide and carbonate radical anion: Two emerging radicals in Biology” [1] to draw attention to these radicals, which had received limited (nitrogen dioxide) or practically no (carbonate radical) attention in the biological literature by then. However, the times were changing due to the work of several researchers around the world. These investigators established that carbon dioxide (produced continuously by cell metabolism) and peroxynitrite (the strong oxidant produced by the extremely rapid reaction between superoxide radical and nitric oxide) react with a considerable second-order rate constant to produce an unstable adduct, nitrosoperoxocarbonate, which undergoes O–O bond homolysis, producing solvent-caged nitrogen dioxide and carbonate radical pairs. Part of these radicals escapes the cage as nitrogen dioxide and carbonate radicals (determined as 0.33 each/peroxynitrite) while the remaining caged radicals decayed to nitrate and carbon dioxide (determined as 0.67 each/peroxynitrite) (Figure 1). Since part of the carbon dioxide is recycled, it can be viewed as a catalyst of peroxynitrite decomposition to radicals. Therefore, nitrogen dioxide and carbonate radicals were likely to play important roles in the biological actions of peroxynitrite. This radical mechanism has passed the test of the years and has attained widespread acceptance, despite occasional attacks by a few researchers, particularly by W H Koppenol and co-workers. The last attack was published in 2018 [2], predicting a yield of nitrogen dioxide/carbonate radical of less than 0.01 (per peroxynitrite) under physiological conditions and, in contrast to the widely accepted view, suggesting that radical generation is inconsequential to peroxynitrite-induced oxidative damage. To avoid the eventual confusion provoked by such claims, a number of researchers actively involved in establishing the radical connection between peroxynitrite and carbon dioxide reviewed the experimental and theoretical evidence for the radical mechanism and critically analyzed the experiments that have led to erroneous conclusions [3]. The authors conclusively show that all confirmed research by several independent groups on the structural and dynamic properties of peroxynitrite support the radical mechanism. Therefore, the radical mechanism, which invokes the intermediacy of nitrogen dioxide and carbonate radical in all carbon dioxide-catalyzed oxidations/nitrations/nitrosations by peroxynitrite, can be confidently used to interpret peroxynitrite biochemistry.
Figure 1. Schematic representation of the reaction between peroxynitrite and carbon dioxide to produce nitrogen dioxide and carbonate radicals, which are quite reactive towards biomolecules (BM).
References
- O. Augusto, M. G. Bonini, A. M. Amanso, E. Linares, C. C. Santos, S. L. De Menezes. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology Free Radical Biology and Medicine, 32(9): 841–59, 2002. | doi: 10.1016/s0891-5849(02)00786-4
- S. Serrano-Luginbuehl, R. Kissner, W. H. Koppenol. Reaction of CO2 with ONOO–: One Molecule of CO2 Is Not Enough Chemical Research in Toxicology, 31(8): 721–30, 2018. | doi: 10.1021/acs.chemrestox.8b00068
- O. Augusto, S. Goldstein, J. K. Hurst, J. Lind, S. V. Lymar, G. Merenyi, R. Radi. Carbon dioxide-catalyzed peroxynitrite reactivity – The resilience of the radical mechanism after two decades of research Free Radical Biology and Medicine, 135: 210–5, 2019. | doi: 10.1016/j.freeradbiomed.2019.02.026
Ohara Augusto, (oagusto@iq.usp.br) Director of CEPID Redoxoma, PhD Professor at Department of Biochemistry,
Institute of Chemistry, University of São Paulo, Brazil
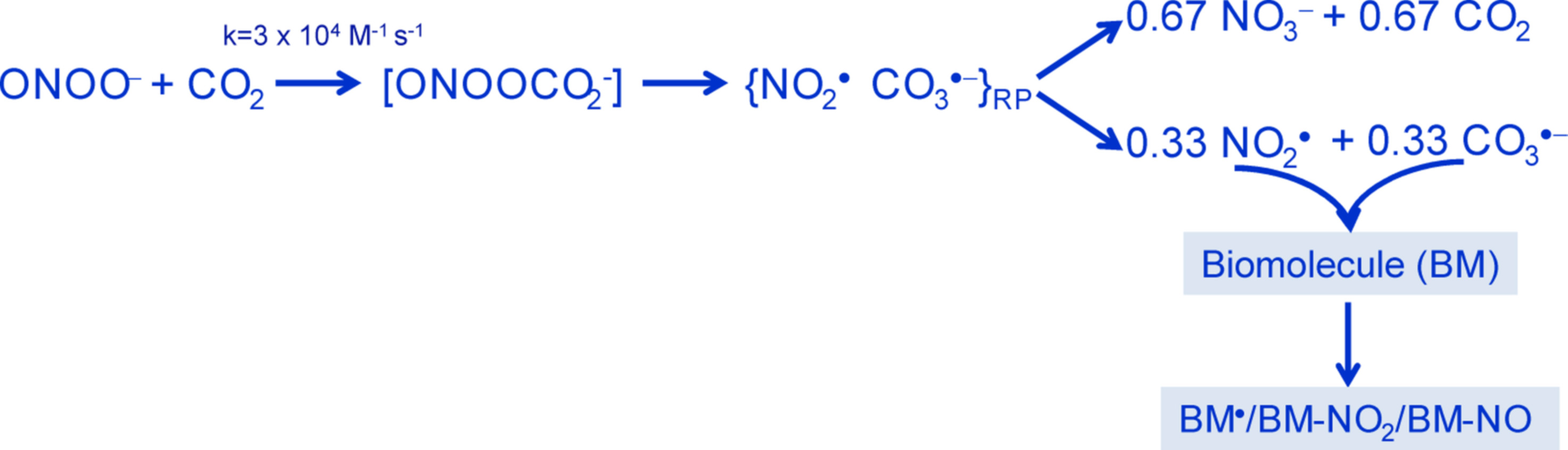
Add new comment