Redoxoma Highlights by Mauricio da S. Baptista
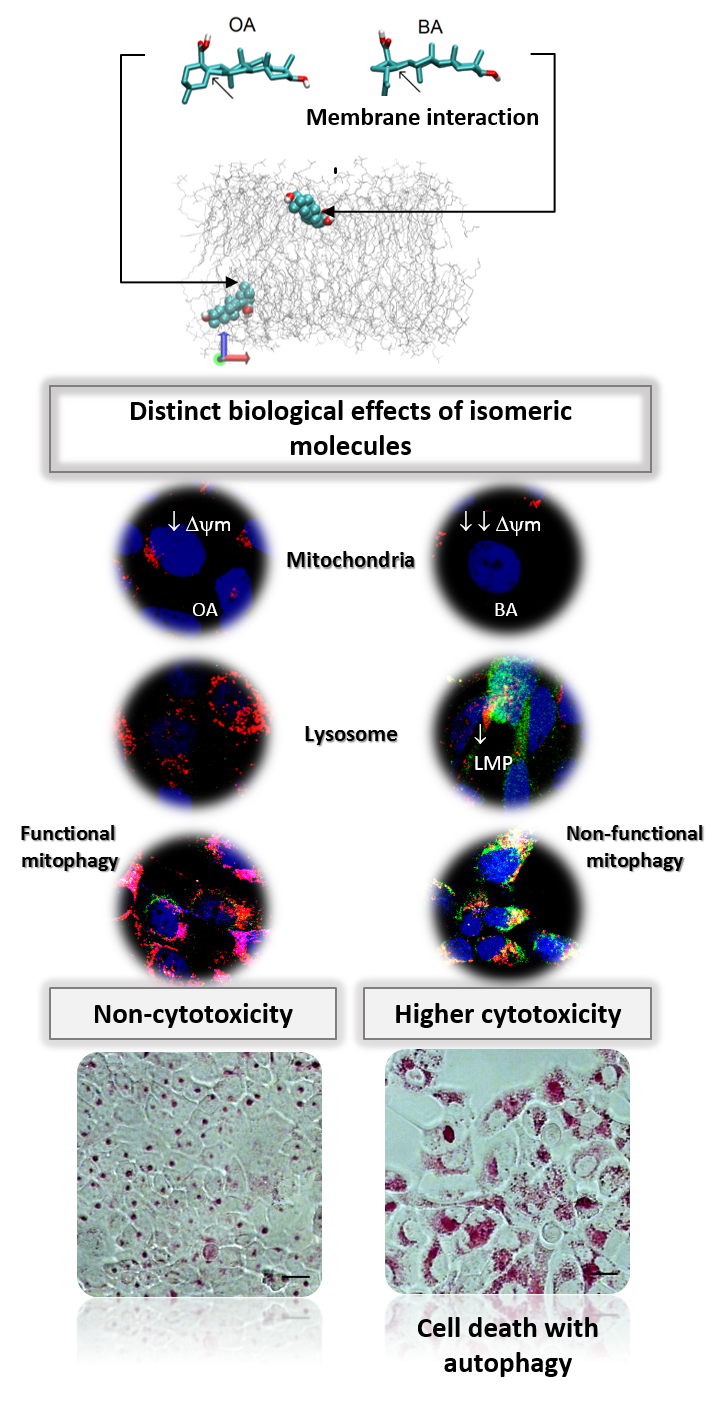
Parallel damage in the mitochondrial and lysosomal membranes leads to cell death with autophagy An important aim of our CEPID-Redoxoma is to develop diagnostic and therapeutic applications of redox processes. In this context, antioxidant therapies are at the frontline of our interests as a group. In parallel, however, a smaller but nonetheless significant group of strategies aim to explore prooxidant and stress-enhancing effects of distinct interventions, mainly to achieve selective toxicity towards damaged or tumor cells. The group of Prof. Mauricio S. Baptisata, from our CEPID-Redoxoma, has been exploring for more than a decade photo-induced compounds as a means to achieve such type of effects. Interestingly, this group recently provided a significant contribution [1] using triterpenoid pentacyclic molecules which are not photo-active, namely Betulinic acid (BA) and Oleanolic acid (OA). In parallel, these results are helping to understand the cellular responses to several photo-induced processes.
Although being chemical isomers, BA and OA have almost identical physico-chemical properties. However, BA is a lot more cytotoxic. The main difference between these two molecules is their efficiency of membrane interaction. BA binds and damages membranes, an effect that is not observed for OA. This membrane-damaging effect of BA associates with parallel damage on mitochondria and lysosome, turning autophagy into a destructive process. Indeed, the higher cytotoxicity of BA correlated with its stronger efficiency in damaging membrane mimics. OA caused mitochondrial but not lysosomal damage, leading to an autophagy response able to rescue cellular homeostasis. In fact, the response to OA was turned into cell death upon concomitant lysosomal inhibition by chloroquine or bafilomycin-A1. Based on these findings, a new mechanism of cell death was proposed, namely, that autophagy will turn into a destructive outcome when there is parallel damage in mitochondrial and lysosomal membranes.
The transition from the pro-survival to the pro-death roles of autophagy still causes intense debate in the scientific community. These data showed that whether autophagy causes cell rescue or cell death seems to depend on the extent of membrane damage. This concept is likely to provide a basis for the development of new drugs against aggressive cancers. An important outcome of these studies are the ongoing experiments in this group, this time using photoactivable drugs, which are indicating a recurrent scenario of cell death with autophagy upon parallel damage in mitochondria and lysosome.
- W. K. Martins, É. T. Costa, M. C. Cruz, B. S. Stolf, R. Miotto, R. M. Cordeiro, M. S. Baptista. Parallel damage in mitochondrial and lysosomal compartments promotes efficient cell death with autophagy: The case of the pentacyclic triterpenoids Scientific Reports, 5: 12425, 2015 | doi: 10.1038/srep12425
Mauricio da Silva Baptista, PhD. Professor at Department of Biochemistry, Institute of Chemistry, University of São Paulo, Brazil
Add new comment