Redoxoma Highlights by Marilene Demasi and Fernanda M. Cunha
Protein degradation is a vital cellular function, needed to enable cells to get rid of misfolded and toxic proteins, to dispose of unused protein components, to control cell cycle and inflammation or simply to demolish the existing cell milieu for metabolic needs or during cell/tissue remodeling. the proteasome (…) is a crucial regulator of cellular protein degradation and homeostasis A major protein complex, the proteasome, composed of several subunits, is a crucial regulator of cellular protein degradation and homeostasis. How the proteasome is regulated and whether it specifically degrades redox-modified proteins is a matter of debate. These mechanisms were put into a critical perspective in a recent review article by Redoxoma investigators (Demasi & da Cunha, 2018).
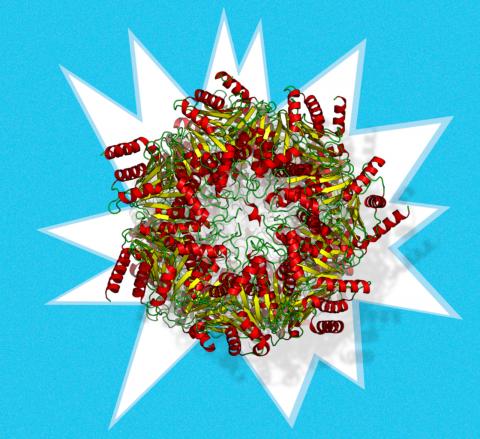
The ubiquitin-proteasome system is the main pathway of intracellular proteins degradation. It requires the conjugation of poly-ubiquitin chains to target proteins, followed by their recognition by the 19S proteasome regulatory unit coupled to the 20S catalytic core particle. Intracellularly, the 20S proteasome stays coupled to its 19S regulatory subunit, which is the most abundant, in addition to the other regulatory units 11S and PA200. ATP is required in the context of protein ubiquitylation, unfolding and translocation into the 20S catalytic chamber, since all such processes are energy-consuming. In addition to the complete assembled particle, a free pool of the 20S catalytic unit has been proposed to occur intracellularly, but its demonstration has been challenging. Existence of this pool was suggested in the 1980's and demonstrated in vitro. By then, it was shown that free 20S catalytic core would be responsible for the degradation of oxidized proteins, thus independently of the 19S regulatory unit and, consequently, of poly-ubiquitylation and ATP. Since then, a number of independent investigators have reported that not only oxidized but also intrinsically disordered proteins can be degraded by free 20S proteasome in vitro.
Despite those evidences, the hypothesis of protein degradation by a free pool of the 20S proteasome in vivo has remained controversial in the literature. protein degradation by a free pool of the 20S proteasome in vivo has remained controversial in the literature We critically reviewed several experimental data that support such hypothesis. From one side of this question, it is clear that participation of the proteasome in cellular proteolysis goes beyond the classical model of protein poly-ubiquitylation. Indeed, protein degradation through the proteasome may proceed through multiple proteasome complexes, with different requirements, a fact that is sometimes overlooked by part of the scientific community. In the case of the free 20S proteasome, its gate opening is a mandatory and crucial event allowing substrate degradation, but there is not much data on what regulates this process. Our analysis concluded that despite the evidences, few contributions produced unequivocal data supporting in vivo proteolysis through the free 20S proteasome, rendering this issue a meritorious focus of further investigation.
Related article:
- M. Demasi, F. M. da Cunha. The physiological role of the free 20S proteasome in protein degradation: A critical review Biochimica et Biophysica Acta (BBA) - General Subjects, 1862(12): 2948–54, 2018 | doi: 10.1016/j.bbagen.2018.09.009
Marilene Demasi (marimasi@butantan.gov.br),
Ph.D. Professor at Laboratory of Biochemistry and Biophysics,
Instituto Butantan, Brazil and
Fernanda M. da Cunha (phernandacunha@gmail.com),
Ph.D. Professor at Department of Biochemistry,
Paulista School of Medicine, Federal University of São Paulo, Brazil
Add new comment